Petrous Apex Meningioma
This is a preview. Check to see if you have access to the full video. Check access
Resection of a Petrous Apex Meningioma via the Supracerebellar Transtentorial Approach
Petrous apex meningiomas are located along the anterior extent of the petrous temporal bone. These tumors originate at or above the internal acoustic canal (IAC) and impinge on cranial nerves (CNs) VII-VIII and the brainstem. As the tumor expands, it can invade the Meckel’s cave and subsequently impinge on CN V and the temporal lobe.
Meningiomas of the posterior petrous bone have been classified into four groups according to the modified Desgeorges and Sterkers classification: posterior petrous, meatus and IAC, petrous apex without invasion of the IAC, and cerebellopontine angle with invasion of the IAC. Most petrous apex meningiomas do not infiltrate the IAC.
Although these tumors are generally benign, the lack of capacitance within the posterior fossa increases the incidence of significant consequences caused by brainstem or cranial nerve compression. It is this proximity to critical structures that makes management of these tumors challenging.
Petrous apex meningiomas are ambiguous entities because they have been classified along with petroclival and cerebellopontine angle meningiomas. Petroclival meningiomas typically displace CN V laterally, whereas petrous apex meningiomas mobilize this nerve medially. I have dedicated a chapter to the discussion of petrous apex meningiomas in addition to the chapters for petroclival and cerebellopontine angle meningiomas.
Clinical Presentation
Based on the complex neurostructural anatomy adjacent to the anterior segment of the petrous bone, presenting signs and symptoms are variable. The clinical spectrum includes headaches, cranial nerve palsies (IV-X), trigeminal neuralgia, facial hypesthesia, hearing loss, vertigo, and long tract signs from pyramidal tract compression.
Evaluation
The typical magnetic resonance (MR) imaging demonstrates the size and location of the mass and estimates it extent of vascularity. The tumor’s relationship to and encasement of the brainstem, cranial nerves, temporal bone, cavernous sinus, and related vasculature are assessed. Edema in the brainstem parenchyma signifies pial invasion and a high risk of new postoperative neurologic deterioration caused by a lack of intact pial dissection planes intraoperatively. Under these circumstances, subtotal decompressive surgery is advised. A computed tomography (CT) scan will identify hyperostosis of the adjacent temporal bone.
A complete preoperative detailed assessment of neurologic function, particularly functions related to the cranial nerves that are likely to be affected, is essential to establish the patient’s baseline functional status. An audiogram is part of this evaluation. The outcomes of these baseline studies, especially those assessing hearing, can impact the selection of the appropriate surgical approach.
For more details about the evaluation of petrous apex meningiomas, please refer to the petroclival meningioma chapter.
Figure 1: Petrous apex meningiomas straddle the petrous ridge. The exuberant vascularity at the base of the tumor is indicated by numerous flow-voids on the T2-wighted sequences (right lower image). Brainstem compression led to imbalance in this patient.
Preoperative Considerations and Selection of the Surgical Approach
The choice of approach for resection of extra-axial petrous apex tumors depends on the location of the predominant bulk of the mass. I typically employ the retrosigmoid/lateral supracerebellar corridor as part of the transtentorial approach. Other options to consider include the subtemporal approach combined with an apical petrosectomy. Large tumors are best exposed via a two-staged operation (posterior fossa followed by subtemporal surgery).
The retrosigmoid suprameatal approach allows petrous apex drilling above CN V and expands the operative corridor further. I favor the posterior fossa corridors with extended modifications into the subtemporal space in order to avoid temporal lobe retraction, especially on the dominant side. However, the extent of supratentorial tumor or compromise and encasement of the surrounding structures can render the subtemporal approach combined with an apical petrosectomy a more strategically appropriate choice.
Preoperative embolization of tumor-feeding vessels can be a reasonable consideration in highly vascular tumors
Intraoperative neuronavigation using MRI and CT (for possible suprameatal osteotomy) is beneficial. Moreover, somatosensory evoked potentials (SSEPs) and brainstem auditory evoked responses (BAERs) monitoring are valuable to facilitate desirable outcomes.
Operative Anatomy
The anatomy of the petrous ridge and the tentorial incisura is discussed in the chapter on petroclival meningiomas. The suprameatal modification of the transtentorial approach may be beneficial for reaching a portion of the meningioma hidden behind the petrous ridge.
The suprameatal osteotomy via the retrosigmoid approach can potentially prove advantageous for exposure of tumors that are primarily within the cerebellopontine angle and that also extend into the middle cranial fossa around the Meckel's cave. This osteotomy may obviate the need for a supratentorial craniotomy. The suprameatal osteotomy is mentioned here for completeness, but I rarely resort to this modification.
Figure 2: The microsurgical anatomy of a right-sided suprameatal approach is briefly reviewed here. The suprameatal tubercle is just above the internal acoustic meatus and posterior to the trigeminal nerve. Upon removal of the dura from the surface of the suprameatal tubercle and drilling the tubercle, additional exposure along the trigeminal nerve and above it is secured. Although helpful, this approach is rarely necessary for petrous apex meningiomas and other related skull base tumors (images courtesy of AL Rhoton, Jr).
Figure 3: A superior view of the petrous apex and cerebellopontine angle is demonstrated. The trigeminal nerve travels medial to the suprameatal tubercle to enter the Meckel's cave. After disconnection of the superior petrosal sinus, the suprameatal tubercle has been drilled to extend access to the anterior portion of the trigeminal root (left lower image). The lateral view shows the enhanced exposure via drilling of the tubercle and opening part of the Meckel's cave (right lower image)(images courtesy of AL Rhoton, Jr).
RESECTION OF PETROUS APEX MENINIGOMA
As mentioned previously, the combined retrosigmoid and supracerebellar transtentorial approach with a potential need for suprameatal drilling of the petrous apex is highly appealing to me because this is the least disruptive and safest approach to the petrous apex as long as the tumor does not extend anteriorly to near the cerebral peduncle.
The anterior reach of this approach along the petrous ridge is limited and the working distance is long. This technically demanding approach for a petrous apex meningioma is discussed in detail below, but further specifics are available in the Paramedian Supracerebellar Transtentorial chapter.
Figure 4: A combined approach may be necessary for large petrous apex meningiomas. When planning the initial incision aimed at the retrosigmoid/supracerebellar route, I take into account the potential need for a subtemporal approach during the same operation. The vertical limb of the incision is initially used.
INTRADURAL PROCEDURE
I present a selection of intraoperative images from the patient in Figure 1 before proceeding with the discussion of technical tenets.
Figure 5: A left-sided retrosigmoid/lateral supracerebellar approach is demonstrated. Note the hypervascularity of the petrous dura (left upper image) and tentorium (right upper image); the superior petrosal vein is transected. The trigeminal nerve is dissected from the inferior pole of the tumor (left lower image). Next, the tumor is devascularized along its base at the tentorium.
Figure 6: The typical location of these tumors in relation to the surrounding neurovascular structures, including CNs V, VII, and VIII, is illustrated.
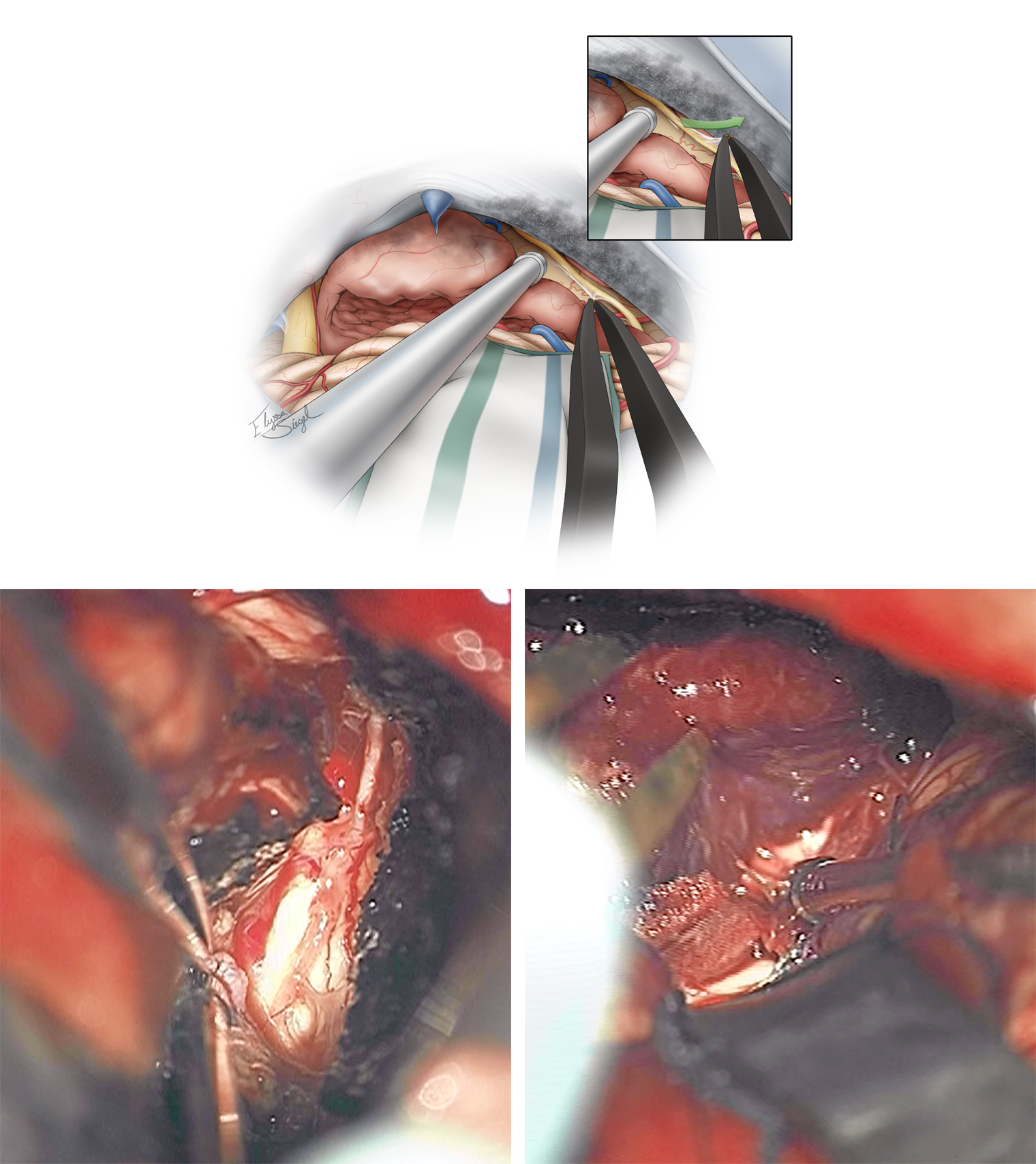
Figure 7: The first important step in managing the tumor involves its devascularization along the petrous dura, petrotentorial junction, and the tentorium. Torrential bleeding from this junction is a common occurrence during mobilization of the tumor (left lower photo). Bipolar coagulation and patience are needed to control the bleeding. The CN IV is displaced anteriorly by the tumor capsule and should be protected and kept out of harm’s way (right lower photo).
Figure 8: Dedressing and devascularization of the tumor is followed by the next “D,” namely decompression of the tumor. An ultrasonic aspirator is used for tumor enucleation. Initial aggressive devascularization at the tumor base significantly facilitates this step. Early identification of the nerves around the tumor capsule, including CNs V, VII-VIII is beneficial for their protection.
Figure 9: Dissection of the tumor capsule from CNs V, VII, and VIII and the brainstem is the next phase of the operation. The encasing arachnoid sheath of the nerve is used as a handle to mobilize the nerve away from the tumor capsule using fine forceps (inset image). This maneuver avoids direct handling of and resultant manipulation injury to the nerve. The arachnoid and pial planes are preserved.
Figure 10: CN IV is found along the tentorial incisura and is very adherent to the anterior tumor capsule. Although preservation of this tiny nerve is possible with meticulous and time-consuming dissection, most large tumors have rendered the nerve nonfunctional and the nerve may be sacrificed without any detectable consequences. Again, the arachnoid sheath of the nerve is used to mobilize the nerve (inset image). The lower photos demonstrate the route of the dissected CN IV (left) and mobilization of the tumor capsule away from the brainstem (right).
Figure 12: The anatomy of the posterior fossa after removal of the tumor in the area is shown. Note the combined retrosigmoid and supracerebellar operative corridor
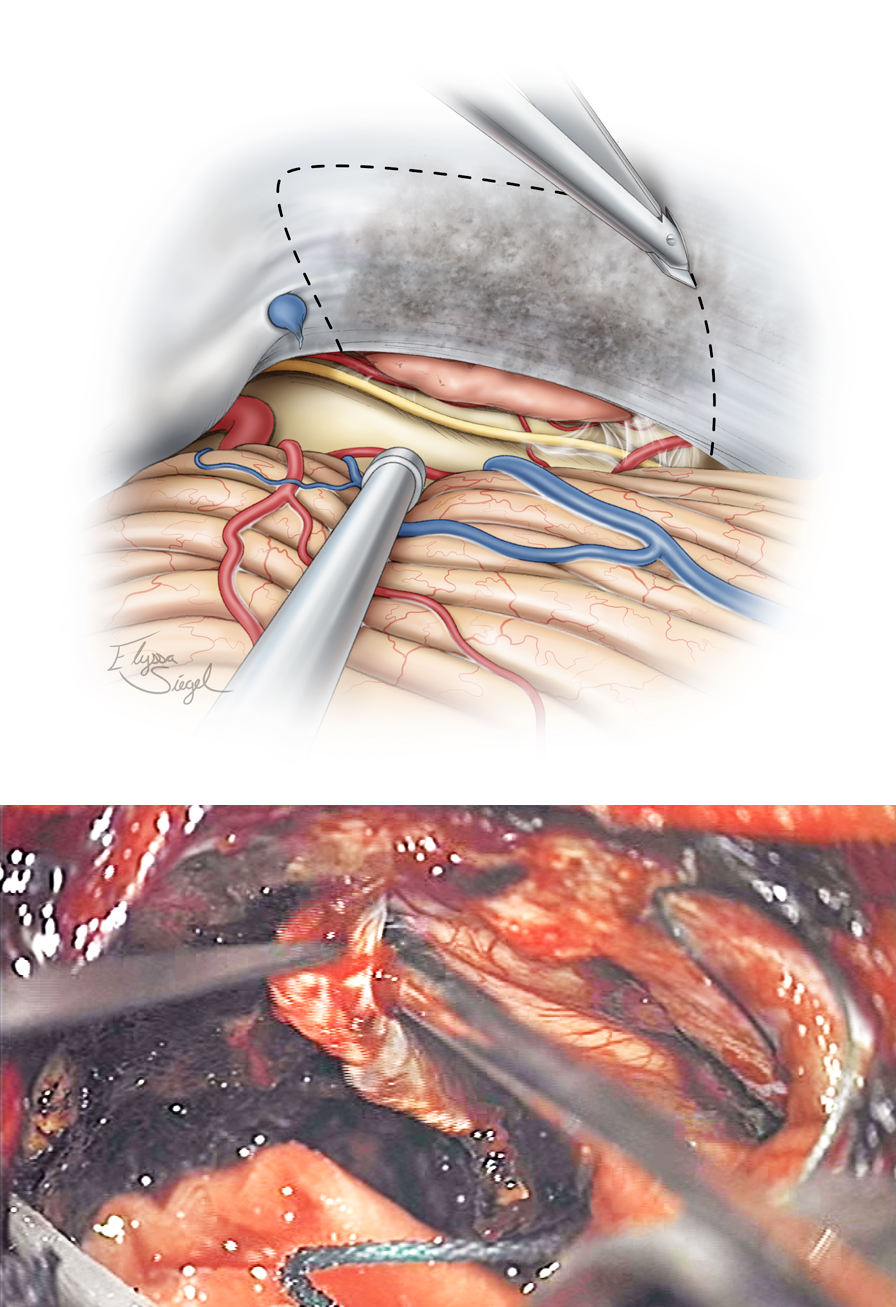
Figure 13: The undersurface of the tentorium is coagulated and a wide window of this structure is incised to expose the supratentorial component of the tumor. The Karlin blade is used to perform the dural incision parallel to the transverse sinus. The lateral incision follows the contours of the petrous ridge while the medial tentorial cut is just lateral to the straight sinus. Venous bleeding from the leaflets of the tentorium is controlled with Gelfoam packing.
Figure 14: Next, the supratentorial portion of the tumor is removed. The anatomy of the final resection cavity is shown. If necessary, the dura over the petrous ridge is reflected; resection of the bone above CN V and toward the petrous apex expands the corridor over the ridge and the lateral middle fossa.
Closure and Postoperative Considerations
The tentorium is not reconstructed. Bone wax is used to occlude the air cells if the petrous apex was partially removed via drilling. Closure is performed in the standard fashion.
Pearls and Pitfalls
- Devascularization of the tumor along the petrous apex and tentorium is the key step in facilitating effective tumor debulking and safe mobilization of the tumor capsule away from the vital neurovascular structures within a relatively bloodless and visible operative field.
DOI: https://doi.org/10.18791/nsatlas.v5.ch05.9
Contributor: Andrew R. Conger, MD, MS
References
Bogaev C, Sekhar LN. Petrovlical meningiomas, in Atlas of Neurosurgical Techniques: Brain, 1st ed. New York: Thieme Medical Publishers, 2006.
Peyre M, Bozorg-Grayeli A, Rey A, Sterkers O, Kalamarides M. Posterior petrous bone meningiomas: surgical experience in 53 patients and literature review. Neurosurg Rev. 2012;35:53-66.
Samii M, Gerganov VM. Cerebellopontine angle meningiomas, in DeMonte F, McDermott MW, Al-Mefty O. (eds): Al-Mefty’s Meningiomas. 2nd ed. New York: Thieme Medical Publishers, 2011.
Please login to post a comment.