Cerebellopontine Meningioma
This is a preview. Check to see if you have access to the full video. Check access
Resection of a Lateral Petrous/Cerebellopontine Angle Meningioma
Please note the relevant information for patients suffering from meningioma is presented in another chapter. Please click here for patient-related content.
Cerebellopontine (CP) angle tumors account for about 10% of all intracranial tumors, and approximately 6 to15% of CP angle tumors are meningiomas. These tumors typically originate from the dura of the posterior surface of the petrous pyramid lateral to cranial nerve (CN) V. Cushing and Eisenhardt originally noted in 1938 that these tumors “simulate acoustic neuromas.”
The point of origin of the tumor establishes the resultant distorted anatomy of the adjacent critical neurovascular structures. Because the origin of most CP angle meningiomas is posterior to cranial nerves (CNs) V, VII/VIII, IX, and X, these nerves are displaced anteriorly and often separated by an intact arachnoid membrane. However, since these structures are within the blind spot of the surgeon and identified late during dissection, they are vulnerable to operative injurious maneuvers. Adjacent, medially displaced vascular structures include the basilar, vertebral, anterior inferior cerebellar, and posterior inferior cerebellar arteries.
In accordance with the inclusive definition of CP angle meningiomas, these lesions demonstrate variability in their clinical presentation, operative challenge, and prognosis. To address this heterogeneity, CP angle meningiomas are classified into premeatal, postmeatal, and combined groups, based on their location relative to the internal acoustic canal (IAC).
The postmeatal group is subclassified based on the extension of the tumor into the IAC. The premeatal group is subdivided into tumors with medial/superior extension (with or without expansion into the Meckel’s cave, the supratentorial space, or the IAC) and those with medial/inferior extension (with or without expansion into the jugular foramen, IAC, or foramen magnum).
These classifications are indicative of surgical challenges and prognosis. More medially located tumors and those involving the Meckel’s cave, IAC, supratentorium, jugular foramen, or foramen magnum predict poorer outcomes, with jugular foramen involvement most strongly associated with operative morbidity. As expected, these factors are predictive of subtotal resection and an increased risk of recurrence.
Diagnosis
CP angle meningiomas present similar to other CP angle lesions. Common symptoms include hearing loss, vertigo, headache, facial pain/numbness, and loss of coordination. Less common symptoms include hemiparesis, myelopathy, hemianesthesia, hoarseness, dysphagia and symptoms of elevated ICP from obstructive hydrocephalus.
Premeatal tumors typically present early with trigeminal neuropathy (facial pain/numbness), mild facial weakness and hearing loss. Postmeatal tumors often grow much larger before presentation and present with cerebellar dysfunction.
Evaluation
Large tumors with inferior extension warrant an otolaryngologic evaluation, including vocal cord and swallowing studies.
Certain CP angle meningiomas can be difficult to distinguish from acoustic neuromas on magnetic resonance (MR) imaging because both of these tumor types homogeneously enhance. However, meningiomas are typically centered away from the IAC and have a broad dural attachment to the posterior aspect of the petrous ridge or tentorium. Meningiomas rarely originate within the IAC, but they can spread into the IAC secondarily. The IAC is usually not expanded in diameter, and the angle between the tumor and the petrous pyramid is wide.
Acoustic neuromas widen the IAC, form an acute angle with the petrous pyramid, and are likely to harbor a cystic component and a cerebrospinal fluid (CSF) cleft on their posterior capsule.
For patients with meningiomas, T2-weighted images are carefully evaluated to determine if pial invasion or encasement of vasculature is present. Intraparenchymal edema indicates violation of pial surfaces and heightens the risk of operative morbidity if aggressive resection is pursued.
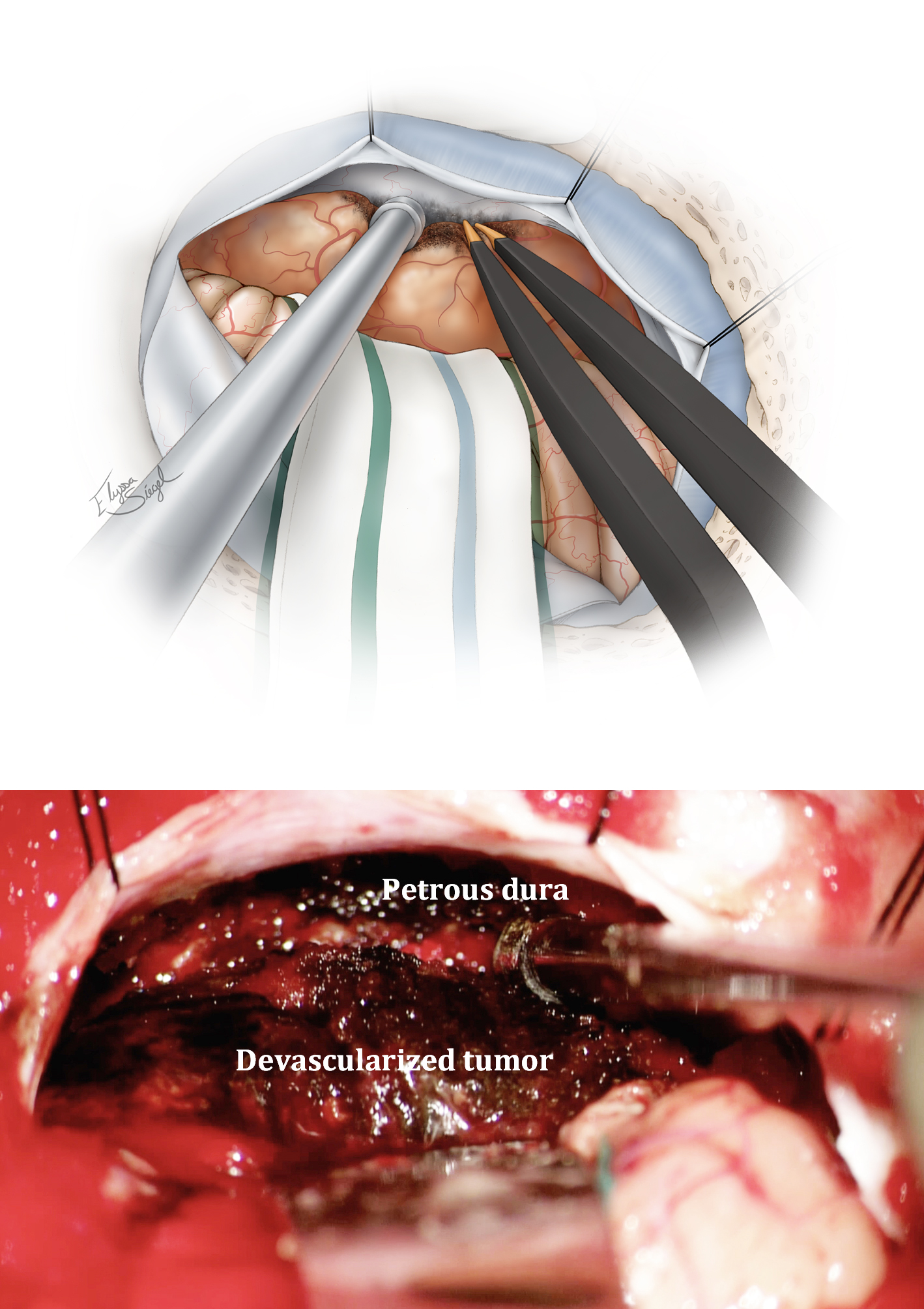
A computed tomography (CT) scan will guide resection of the hyperostotic bone. Calcified tumors present a daunting challenge because of their adherence to the surrounding structures and resistance to debulking and mobilization during surgery. I do not advocate embolization even for large tumors because they can be readily devascularized at their base along the petrous bone early in surgery.
Figure 1: A left-sided petrous/CP angle meningioma (top row) is shown. Petrous meningiomas are typically centered away from the IAC and have a broad attachment to the posterior aspect of the petrous dura. The images in the lower row demonstrate a right-sided jugular foramen meningioma without a significant intracanalicular extension.
Indications for Procedure
Management options for meningiomas include observation with serial imaging, surgical resection, stereotactic radiosurgery (SRS), and a combination of resection and SRS. Small asymptomatic or minimally symptomatic tumors in older patients or those with medically prohibitive conditions may be observed with repeat imaging. Treatment is indicated for growing tumors. Small growing tumors without mass effect may be treated with SRS.
Tumors causing neurologic dysfunction secondary to mass effect are resected with the goal of complete tumor removal. However, gross total resection should not compromise neurologic function. Subtotal resection to preserve function followed by SRS for a residual growing tumor is an emerging paradigm for treatment of complex skull base meningiomas.
I prefer the goal of gross total resection and accept subtotal resection to preserve function, based on the intraoperative findings. I then monitor the residual tumor by serial imaging. I reserve SRS for any growth of the residual tumor seen on follow-up imaging.
Preoperative Considerations
Once a decision has been made to proceed with resection, intraoperative monitoring of brainstem auditory evoked responses (BAERs) and somatosensory evoked potentials (SSEP) is recommended. Facial nerve electromyography and lower CNs monitoring is advisable for patients with tumors infiltrating the IAC or jugular foramen.
I install a lumbar drain at the beginning of the procedure to relieve infratentorial tension. This technique will allow early and safe tumor mobilization (without its decompression) so that the base of the tumor along the petrous pyramid can be thoroughly devascularized. This maneuver enhances operative efficiency during tumor decompression and dissection.
Intraoperative navigation based on a high-resolution CT of the skull base is beneficial for resection of the affected bone.
Operative Anatomy
A detailed understanding of the relevant anatomy of the CNs within the posterior fossa, as well as the anterior and posterior inferior cerebellar arteries, is necessary.
Click here to view the interactive module and related content for this image.
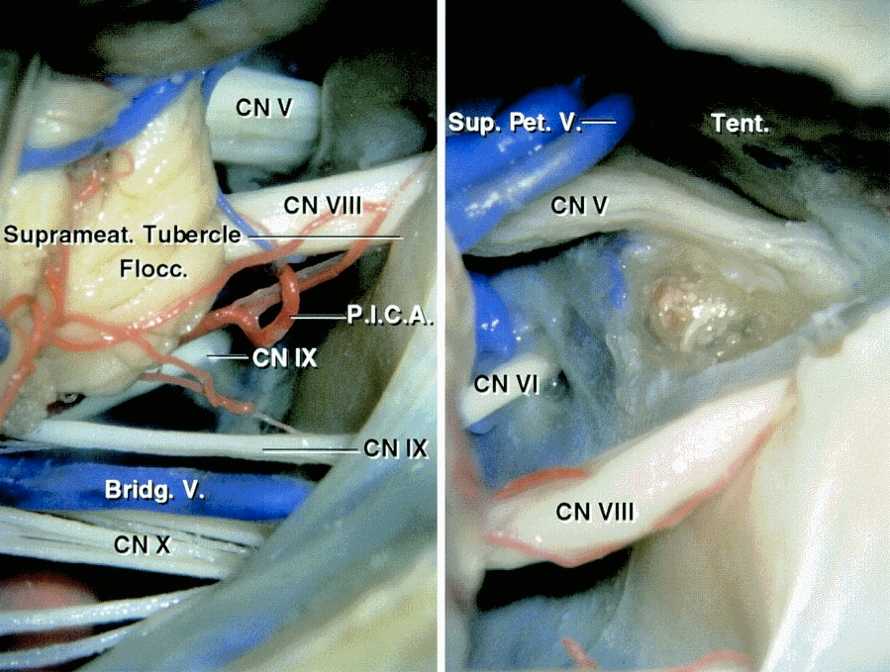
Figure 2: Cranial nerves of the CP angle through a left-sided retrosigmoid approach (left image) are indicated. The suprameatal tubercle was partially removed following transection of the tentorium (right image). The transtentorial suprameatal corridor facilitates removal of meningiomas extending into the middle fossa and the Meckel’s cave through the retrosigmoid route (images courtesy of AL Rhoton, Jr). For more information, please refer to the chapter on petrous apex meningiomas. Most CP angle meningiomas displace the lower cranial nerves inferiorly and CNs V-VIII anteriorly.
Click here to view the interactive module and related content for this image.
Figure 3: Additional detailed anatomy of the cranial nerves within the posterior fossa is illustrated.
RESECTION OF CEREBELLOPONTINE ANGLE MENINGIOMA
Several different skull base approaches for handling CP angle meningiomas have been explored, including the retromastoid, translabyrinthine, retrolabyrinthine, transpetrosal, far lateral, and middle cranial fossa corridors. The final choice of surgical approach depends on the specific features of the tumor, including its site of origin, location of the bulk of the tumor in relation to the neurovascular structures and specifically the cranial nerves, and the patient’s preoperative neurologic function.
For larger tumors in patients without serviceable hearing, the translabyrinthine approach and its variations can be considered; however, there is evidence to support the theory that preoperative CN VIII dysfunction caused by a meningioma is more likely to improve than is the dysfunction caused by an acoustic neuroma. I believe this approach has a minimal role for resection of CP angle meningiomas and often leads to unnecessary exposure and lengthy operative sessions.
I prefer the extended retromastoid approach for almost all CP angle meningiomas. This approach provides excellent visualization of the CP angle with low approach-related morbidity in comparison with the more extensive skull base osteotomies. CP meningiomas rarely extend along the ventral brainstem and engulf the cranial nerves. This is particularly true for postmeatal tumors. Premeatal tumors require the surgeon to work through multiple corridors between various cranial nerves; this limitation of the working space is not altered by more extensive petrosal osteotomies.
Tumors extending to the foramen magnum can be approached by inferior extension of the retrosigmoid osteotomy. Similarly, the tumors with significant supratentorial component can be resected through a combined retro-, supracerebellar corridor supplemented by expansion of the operative space into the middle fossa through tentorial sectioning (see Transtentorial Approach for Parahippocampal Lesions).
INTRADURAL PROCEDURE
After the retromastoid craniotomy is complete and the dura is opened and reflected, microdissection begins. The following steps, in their specific order, improve the safety and efficacy of meningioma resection (also known as the 4Ds of meninigomas): 1) Dedress (expose) the tumor, 2) Devascularize the tumor, 3) Debulk the tumor, and finally 4) Dissect the tumor. As mentioned above, maximal devascularization before debulking avoids excessive blood loss. It also allows efficient tumor debulking and, importantly, a relatively bloodless, pristine operative field during dissection of the brainstem and cranial nerves.
The following illustrations summarize the techniques for resection of a postmeatal tumor.
Figure 4: Initial intradural exposure or Dedressing of a left-sided tumor is indicated. Note the bone removal over the sigmoid sinus to reflect the sinus away from the operative trajectory using dural retraction sutures. If a lumbar drain is not available, CSF may be released through the CP angle cisterns around the lower CNs. The arachnoid adhesions between the cerebellum and posterior tumor capsule are disconnected.
Figure 5: The approximate distribution of the cranial nerves in relation to the anterior capsule of a postmeatal tumor is shown. A careful study of the preoperative MR images should provide the surgeon with a 3D understanding of the pathoanatomy of the mass and the location of vital structures.
Figure 6: Next, I coagulate and devascularize the posterior tumor capsule. It is important to protect the en passage vessels en route to the cerebellum.
Figure 7: The next important maneuver involves devascularization of the tumor attachments and interruption of the arterial feeders along the lateral petrous apex without tumor debulking. I maximally disconnect the tumor along this plane, but pay special attention to avoid injuring the cranial nerves entering their corresponding foramina along the anterior tumor capsule. CSF drainage should allow gentle tumor mobilization without undue retraction on the surrounding structures. The petrous-tentorial junction is identified and serves as a landmark for surgical orientation. The lower cranial nerves may be visible along the lower pole of the medium size tumors.
Figure 8: Next, I debulk the tumor using an ultrasonic aspirator. The devascularized tumor can now be enucleated efficiently since the need for stepwise hemostasis within the tumor is minimized. The wall of the anterior capsule should be left intact and not inadvertently violated.
Figure 9: Adequate tumor enucleation allows flexible tumor capsule mobilization without undesirable traction on normal structures. The inferior pole of the capsule is now sharply dissected away from the lower cranial nerves. Arachnoid membranes over the nerves are carefully respected. The adherent vessels are microsurgically released.
Figure 10: Next, the superior pole of the tumor is mobilized away from the tentorium and CN V. The superior petrosal vein is sacrificed. I look for CN IV (encased in a thick arachnoid layer) and ensure its preservation without its direct manipulation. The en passage branches of the superior cerebellar artery are protected.
Figure 11: Cottonoid patties are used to “wipe” the lateral cerebellum away from the mobilized tumor and mark the corresponding dissection planes. The safest vector of tumor mobilization/”rolling” is from medial to lateral so that the cranial nerves are identified early near their roots at the level of the brainstem (upper image). The middle, intraoperative photo, demonstrates this medial-to-lateral dissection maneuver; the root exit zone of CN VII is identified (arrow). The “tumor feeding arteries” are carefully pursued to the level of the capsule and only then are sacrificed (lower image, arrow-CN VII is marked with *). This technique ensures preservation of the en passage vessels including the labyrinthine artery.
Figure 12: Tumor-feeding arteries originating from the superior cerebellar artery branches are also clearly identified, traced to the tumor, and then sacrificed. The tumor is then sharply dissected from the origin of CN V and the lateral pons. The pial veins on the anterior surface of the pons are preserved. Further stepwise tumor debulking is necessary to allow tumor mobilization without placing vital structures under the risk of pressure injury.
Figure 13: Finally, the tumor capsule is carefully inspected to ensure all its attachments are disconnected. It is then removed piecemeal. The CN V can be quite adherent to the nerve requiring meticulous dissection to avoid trigeminal neuropathy. All the pial planes, covering the brainstem, should be maintained. Pieces of papaverine-soaked Gelfoam are used to relieve vasospasm in the small vessels on the cranial nerves and brainstem.
Figure 14: Before closure, all of the critical neurovascular structures are inspected to confirm their anatomical preservation. The dura of the petrous bone is resected or coagulated to decrease the risk of tumor recurrence; this maneuver should not cause heat injury to the cranial nerves as they enter their corresponding foramina. The affected section of the petrous bone may be drilled.
CP Angle/Tentorial Meningioma
Resection of premeatal tumors follows the same microsurgical techniques. However, the cranial nerves are displaced posteriorly and the surgeon must work between the cranial nerves to remove the tumor piecemeal. Cranial nerve V is most tolerant of mobilization, and the working windows above and below this nerve (supra- and infratrigeminal windows) should be exploited. Other working windows are between CNs VII/VIII complex and the lower cranial nerves. The tumor frequently widens these interneural corridors. The dissection of the tumor is conducted in the lateral to medial direction.
The cranial nerves and vascular structures that are embedded in the tumor should be carefully preserved. Some portion of the premeatal tumors is routinely left behind to achieve this goal as the limited working space between the cranial nerves frequently precludes gross total removal.
Both pre- and postmeatal meningiomas can extend into the IAC. Exposure of the intrameatal part of these tumors requires unroofing of the posterior wall of the IAC. The extent of bone drilling is determined by the extension of the tumor into this canal.
Lateral Cerebellar Meningioma
Finally, the techniques of resection for cerebellar convexity meningiomas are the same as the ones for postmeatal tumors. A preoperative MR venogram establishes the patency of the transverse and sigmoid venous sinuses and estimates the feasibility of gross total tumor removal if the affected venous sinus is occluded. Partially occluded sinuses are not removed or manipulated and their residual tumor is assessed via surveillance imaging; growing residual tumors undergo radiosurgery.
Figure 15: A lateral cerebellar meningioma with occlusion of a segment of the transverse sinus is shown. Note the component of the tumor filling the sinus (left image, arrow). MR venogram confirmed occlusion of this focal segment. This tumor was resected and the affected length of the dural sinus removed via drilling of the bone above and below the transverse sinus. Two silk sutures were used to disconnect the tumor-infiltrated segment from the patent segments. The entry point of the vein of Labbe was preserved.
Petrosal Meningioma
Closure
All the petrous and mastoid air cells are thoroughly waxed and obliterated. The closure is conducted in the standard fashion.
Postoperative Considerations
Posterior fossa surgery can result in transient obstructive hydrocephalus and chemical meningitis; therefore, the patient is watched closely for evidence of these conditions.
Chemical meningitis is treated with a short course of steroids, and transient communicating hydrocephalus often requires serial lumbar punctures or ventriculostomy for CSF diversion. The obstruction of CSF pathways is usually transient, but permanent CSF diversion may be occasionally necessary.
Pearls and Pitfalls
- The tumor should be dedressed (exposed), devascularized, debulked, and dissected, in that order.
- Preservation of the arachnoid and pial membranes covering the cranial nerves and brainstem is paramount for a desirable outcome.
- If the tumor is very adherent along one location/plane, it is best to move to another more defined tumor-brainstem/CN interface and continue dissection toward the first location.
- I always look out for cerebrovascular structures along the anterior tumor capsule (within my blind spot). I would rather say “there it is” and be wrong 1000 times, than say “there it was” and be right once.
DOI: https://doi.org/10.18791/nsatlas.v5.ch05.10
Contributor: Andrew R. Conger, MD, MS
References
Agarwal V, Babu R, Grier J, Adogwa O, Back A, Friedman AH, et al. Cerebellopontine angle meningiomas: postoperative outcomes in a modern cohort. Neurosurg Focus. 2013;35: E10.
Al-Mefty O. Operative Atlas of Meningiomas. Philadelphia: Lippincott-Raven, 1998.
Cushing H, Eisenhardt L. Meningiomas: Their Classification, Regional Behaviour, Life History and Surgical End Results. Springfield, IL: Charles C Thomas, 1938.
Park SH, Kano H, Niranjan A, Flickinger JC, Lunsford LD. Stereotactic radiosurgery for cerebellopontine angle meningiomas. J Neurosurg. 2014;120: 708-715.
Samii M, Gerganov V. Cerebellopontine angle meningiomas, in: Demonte F, McDermott M, Al-Mefty O, (eds): Al-Mefty’s Meningiomas. 2nd ed. New York: Thieme, 2011, 262-269
Samii, M. Cerebellopontine angle meningiomas (posterior pyramid meinigiomas), in: Al-Mefty O (ed): Meningiomas. New York: Raven Press, 1991, 503-515.
Rhoton A. The cerebellopontine angle and posterior cranial fossa nerves by the retrosigmoid approach. Neurosurgery. 2000; 47: 93-129.
Tew JM, van Loveren HR, Keller JT. Atlas of Operative Microneurosurgery. Philadelphia: Saunders, 1994-2001.
Please login to post a comment.