Insular Tumors
This is a preview. Check to see if you have access to the full video. Check access
Dominant Insular Glioma: Strategies for Tumor Removal
The insula plays an important role in visceral sensorimotor processing, sympathetic control of cardiovascular tone and somatosensory input. It also participates in pain processing, motor planning, volitional swallowing, and gustatory, auditory, vestibular, emotional, and cognitive functions, including language. For further information regarding the complex functions of insula, please see Wikipedia.
Compared with other gliomas, insular gliomas are unique in their presentation and behavior. These tumors usually arise in areas of white matter adjacent to the allocortex or mesocortex. During their initial phases of growth, these tumors frequently respect the neocortices, central nuclei, and ventricles.
The insula is a challenging structure to reach surgically because of its complex anatomy and the overlying highly eloquent cortices and white matter tracts, as well as critical vascular structures. The development of microsurgical techniques and implementation of cortical and subcortical mapping strategies under conscious sedation (awake) and general anesthesia (sleep) have advanced the safety of insular surgery.
Diagnosis
The paralimbic region, including the insular lobe, is a common location for gliomas. Up to 25% of low-grade and 10% of high-grade gliomas are found in this region. The clinical presentation of insular gliomas is somewhat associated with their grade.
Low-grade gliomas can grow to a very large size while causing minimal symptoms. Their most common presentation is new onset seizures. The semiology of insular epilepsy reflects its complex functional anatomy. It may mimic temporal lobe epilepsy or nocturnal hypermotor epilepsy of frontal lobe origin. Simple partial seizures are common, with features such as respiratory, viscerosensitive, or oroalimentary symptoms, facial paresthesias, laryngeal constriction, gustatory illusions, hypersalivation with postictal facial paresis, auditory hallucinations, or sensory aphasia.
Because of the paucity of symptoms in their early stages, these tumors can reach an impressive size, while the patient has no apparent neurologic deficit and only unspecific symptoms such as headaches, fatigue, subtle speech difficulties, and behavioral changes.
High-grade gliomas frequently cause surrounding vasogenic edema with tissue infiltration, resulting in local and hemispheric mass effect as well as sensorimotor and language deficits. In most cases, neuropsychological assessment reveals a cognitive decline, most likely as a result of regional edema or seizures.
Evaluation
Magnetic resonance imaging (MRI) with T2-weighted and fluid-attenuated inversion recovery (FLAIR) sequences best delineates the extent of tumor infiltration, which can be limited to the insular lobe or reach the perisylvian opercula and other paralimbic areas, namely the orbitofrontal and temporopolar territories. Diffuseness of the tumor affects its resectability.
Functional MRI (fMRI) and magnetoencephalography can help define the overlying cortical distribution of language, auditory, sensory, and motor function. Diffusion tensor imaging and tractography delineate the underlying major tracts surrounding the insula. Merging three-dimensional cortical and subcortical anatomic functional maps provides highly relevant information for surgical planning. All of this information is supplemented by intraoperative cortical and subcortical stimulation data in order to create adequate operative trajectory to maximize tumor removal while preserving function.
Tumors with higher grade histology often exhibit nodular or global contrast enhancement. Careful attention must be paid to the enhancing areas so that they are biopsied during surgery to permit appropriate tumor grading.
Figure 1: These images in the top row show two different patterns of insular low-grade gliomas. The upper images show the different degrees of diffuseness for tumor margins. Adequate resection of diffuse insular tumors can be very challenging and risky. Functional MRI (fMRI) and diffusion tensor imaging with fiber tracking can estimate the degree of involvement of the functional cortices and white matter tracts (lower images). In the left lower image, the motor speech area overlying the tumor is highlighted. Along with intraoperative mapping data, the fMRI information can guide the surgeon in creating safe transcortical corridors to achieve adequate tumor exposure in large tumors.
Indications for Surgery
In the past, insular gliomas were traditionally managed conservatively and debulking surgery was performed only in patients with neurologic deficits secondary to significant mass effect or edema. However, advances in microsurgical and mapping strategies have now improved the safety of resection and have expanded the indications for patients without preoperative dense neurological deficits.
More recently, the operative paradigm has broadened with a better understanding of the natural history of low-grade gliomas. Despite their initially indolent subclinical infiltration, continuous tumor growth and malignant transformation of these tumors is highly likely.
Despite the lack of Class I evidence, several recent studies support the role of the extent of resection as one of the main prognostic factors in overall survival, progression-free survival and malignant transformation. Maximal tumor resection, while minimizing neurologic deficits, should be the goal in insular glioma surgery.
Furthermore, accumulated experience with resection of these tumors has proven that there is significant functional plasticity in this region of the brain. This plasticity allows most patients to compensate and recover over time after aggressive insular resection, as long as the essential language and motor areas identified by intraoperative cortical and subcortical stimulation mapping were preserved.
In addition to its oncological impact on patient survival and malignant transformation, tumor resection has also proven valuable for advancing the quality of life in patients who present with intractable epilepsy, a very common occurrence with these tumors.
My algorithmic approach to insular gliomas involves surgical resection of suspected low-grade tumors using microsurgical and mapping techniques. However, I approach insular high-grade tumors more conservatively. I believe the risks associated with the resection of insular glioblastoma (especially medially located tumors) is significant despite the use of our current techniques. Unless a high-grade tumor is located along the lateral aspect of the nondominant insula in a young highly functional patient, judicious use of an aggressive operative strategy for insular glioblastoma is advised.
Preoperative Considerations
Hemisphere dominance must be established before deciding on resection using conscious sedation or “awake” versus general anesthesia or “sleep” stimulation mapping. Left-handed patients should have their speech dominance confirmed via fMRI. Patients with extensive frontal extension of their tumor should undergo an fMRI to guide speech mapping in expectation of an inferior frontal partial gyrectomy to increase the working space to remove the superior and anterior poles of the tumor. The transsylvian operative corridor is usually not adequate for removing moderate to large size lesions.
I prefer to resect both dominant and nondominant insular tumors under awake conditions. This method allows more precise and efficient mapping of the intimately involved functional cortices and the white matter tracts while mapping data is unaffected by anesthesia. Moreover, I can examine the patient frequently, and this instant feedback is very assuring, especially during handling of the posteromedial deep margins of the tumor intimately associated with the internal capsule.
In my experience, the patient’s continuous feedback has allowed me to conduct more aggressive resections without incurring additional neurologic morbidity or significant patient discomfort. The operation should be conducted expeditiously as the most cooperative patients lose their patience after about four hours of awake surgery. Because of the technical complexity of this potentially lengthy operation, most colleagues opt to perform sleep mapping.
If necessary, non-dominant tumors may be managed under general anesthesia using sleep mapping techniques to maximize the patient’s comfort and safety of microsurgery. Anxious patients may also be candidates for this methodology. In these instances, mapping protects the descending motor pathways and cortical face area if inferior frontal resection is necessary to reach large tumors.
The lateral lenticulostriate arteries determine the most medial extent of resection. Some operators recommend a preoperative angiogram to more precisely localize these important perforators. I have not required this study because intraoperative inspection often exposes these fine vessels that can be carefully protected.
Operative Anatomy
The insular lobe is part of the cerebral cortex, and covered by the opercula of the frontal, parietal, and temporal lobes. The three-dimensional structure of the insula constitutes a pyramid, and its apex is the most lateral and superficial point of the structure, located 9-16 mm from the cortical surface.
The insular apex is beneath the anterior Sylvian point, just inferior to the vertex of the pars triangularis. The anterior Sylvian point is the most generous portion of the Sylvian cistern where the Sylvian split can begin. The insular cortex is circumscribed by the anterior, superior, and inferior peri-insular sulci. The superior and inferior peri-insular sulci are important surgical landmarks; exposure at their base early in surgery ensures an adequate operative reach to the inferior and superior borders of most lateral insular tumors.
Click here to view the interactive module and related content for this image.
Figure 2: The natural upward retraction of the apex of the pars triangularis leads to the widest section of the Sylvian cistern (A). The lower and upper portions of the frontal and temporal opercula have been removed (B). The central insular sulcus courses superficial to, and almost parallel with, the central sulcus on the convexity. The remaining part of the frontoparietal operculum has been removed (C). The anterior limiting sulcus is directed upward and anteriorly to form the anterior border of the insula. Enlarged view of the insula (D). The frontoparietal and temporal opercula and the upper portions of the central core of the hemisphere have been removed using transverse cuts that extend along the superior and inferior limiting sulcus to show the relationship between the insula and the deeper structures (E). The central core of the hemisphere, which contains the caudate and lentiform nuclei, thalamus, and internal capsule, is located deep in the insula. Enlarged superolateral view of the same specimen is shown in image F to depict the anatomy of deep white matter structures. Limen Ins = limen insula (Images courtesy of AL Rhoton, Jr).
The sulcal-gyral anatomy of the insular cortex is detailed in Figure 2. Two important anatomic landmarks of the insular lobe are the insular stem, which is the anterobasal portion of the insula located in the depth of the proximal Sylvian fissure, and the limen insula, located within the insular stem.
Located medially and deep to the insula are several critical structures. The extreme capsule, claustrum, external capsule, and striatum are located deep to the central portion of the insula. The fibers of the motor cortex converging into the posterior limb of the internal capsule run immediately deep to the posterior segment of the superior peri-insular sulcus. The uncinate fasciculus runs medial to the anterior portion of the superior peri-insular sulcus.
Figure 3: The arteries of the insula originate from the M2 segment. The insulo-opercular or long perforating arteries (arrows) supply the insula and operculum. Abbreviations: alg = anterior long insular gyrus; asg = anterior short insular gyrus; cis = central insular sulcus; ia = insular apex; it = inferior trunk of M2; msg = middle short insular gyrus; P2 = ambient segment of the posterior cerebral artery; plg = posterior long insular gyrus; psg = posterior short insular gyrus. I = olfactory nerve; on = optic nerve; 1 = lateral orbitofrontal artery; 2 = prefrontal artery; 3 = precentral artery; 4 = central artery; 5 = anterior parietal artery; 6 = posterior parietal artery; 7 = angular artery; 8 = temporooccipital artery; 9 = posterior temporal artery; 10 = middle temporal artery; 11 = anterior temporal artery; 12 = temporal polar artery. From Türe U, Yaşargil MG, Al-Mefty O, Yaşargil DC. Arteries of the insula. J Neurosurg. 2000; 92: 676–687.
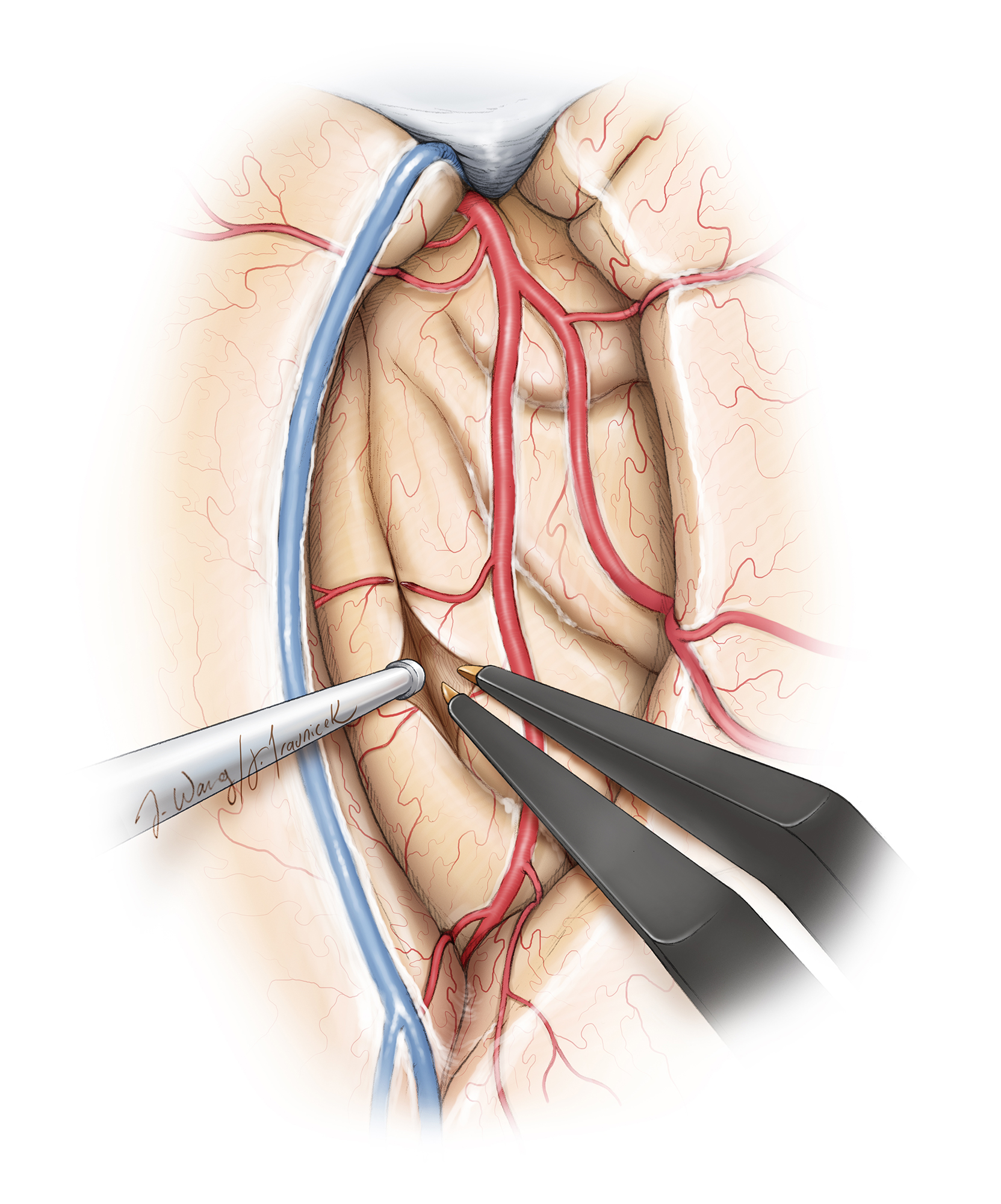
Figure 4: Topography and operative vascular relationships for insular tumors. Note how the lateral lenticulostriate arteries originate from the M1 segment and represent the medial limit of resection. Short M2 perforators supply the tumor. Long M2 perforators may travel through the tumor, but most likely supply corona radiata (especially the ones travelling toward the central sulcus) and must be preserved (upper image). The superior and inferior peri-insular sulci represent the lateral superior and inferior anatomic margins of the resection (lower image,) and their exposure ensures adequate Sylvian fissure split so that residual tumor does not hide within the blind spots of the operator.
The course of the middle cerebral artery (MCA) along the insular surface poses certain technical challenges during surgery. The insula receives most of its vascular supply from short perforating vessels originating from the M2 and M3 segments of the MCA. These short perforators, often engulfed by the superficial aspects of the tumor, can be safely coagulated and cut during subpial resection, effectively devascularizing the tumor. The M2 segments also give rise to long perforating branches that travel posteriorly and superiorly on the insula and supply the corona radiata. These branches must be preserved to avoid ischemic injury resulting in hemiparesis. Careful vascular dissection and preservation of en passage vessels is important.
Figure 5: An inferior view of the left MCA in the region of the anterior perforated substance. Note how the lateral lenticulostriate arteries originate from the M1 segment as it bifurcates into the M2 branches (asterisk). Abbreviations: I = olfactory nerve; III = oculomotor nerve; A1 = precommunicating segment of the anterior cerebral artery (ACA); ach = anterior choroidal artery; ICA = internal carotid artery; it = inferior trunk of M2 segment; li = limen insula; M1 = sphenoidal segment of the MCA; on = optic nerve; ot = optic tract; P2 = ambient segment of posterior cerebral artery (PCA); pco = posterior communicating artery; st = superior trunk of M2 segment; tb = temporal branch of the MCA. From Türe U, Yaşargil MG, Al-Mefty O, Yaşargil DC. Arteries of the insula. J Neurosurg. 2000; 92: 676–687.
The M1 segment gives origin to the lateral lenticulostriate arteries (LLAs) as they course under the anterior perforated substance to emerge on the limen insula. The number of LLAs can vary from only 1 to up to 15. These arteries supply the basal ganglia and internal capsule, but in cases of large tumors, they can be a source of vascular supply to the insula along with the M2-M3 short perforating branches. Early identification of the LLAs is key to avoiding ischemic injury to the medial structures. Dissection of the proximal M1 segment allows identification most lateral LLAs.
MICROSURGICAL RESECTION OF INSULAR TUMORS
A mentioned above, I prefer an awake craniotomy for all insular tumors. The following sections describe the details of the procedure.
Figure 6: Patient positioning under conscious sedation and local anesthesia for resection of a large left insular glioma. I place the single pin of the skull clamp on the mastoid area to have full access to the left posterior temporal territories. The extent of the craniotomy is generous to allow exposure and mapping of the relevant functional cortices.
The patient is positioned supine on the table with the ipsilateral shoulder elevated on a gel roll and the head turned 30 degrees contralateral to the surgical side. I apply some degree of head extension to facilitate access to the superior portion of the insula under the frontoparietal operculum. This head position facilitates Sylvian fissure split as it mobilizes the opercula under the pressure of gravity and provides a more accessible trajectory toward the most posterior portion of the insula. If the head is turned excessively, the patient is uncomfortable and access to the posterior extent of the tumor can be limited as the temporal opercula will obstruct the operative working angle.
Ultimately, the degree of head rotation and the rest of the patient’s position are adjusted primarily for the patient’s comfort. Before placing the skull clamp, 0.5% lidocaine with epinephrine and 0.25% bupivacaine in a 1:1 proportion is used to infiltrate the trajectories of the supraorbital, supratrochlear and occipital nerves, the incision line, the root of the temporalis muscle, and the pin sites.
This method of regional scalp anesthesia provides additional patient comfort. Intraoperative stereotactic navigation is routinely used. Once resection begins, the reliability of navigation diminishes due to brain shift, especially for more lateral structures within the resection cavity. Please refer to the chapters on Language Mapping and Sensorimotor Mapping for Glioma for more details about the initial stages of operative preparation.
A “trauma flap” or question mark incision is employed. This incision allows access to the entire length of the Sylvian fissure and permits mapping of the language and sensorimotor cortices, as necessary. The posterior extension of the craniotomy can be tailored based on neuronavigation data. Once the scalp flap is reflected, additional anesthetic infiltration of the temporalis muscle is necessary.
INTRADURAL PROCEDURE
Sylvian Fissure Dissection
The patient is moderately sedated during the craniotomy and Sylvian fissure dissection. The process of reawakening should take place after the Sylvian fissure has been generously split, the superior and inferior peri-insular sulci identified, and the lateral portion of the tumor through the transsylvian route removed.
The Sylvian fissure is split using the “inside-to-outside” technique. The distal part of the fissure harboring the generous Sylvian cistern is split, and this dissection is extended to the depth of the fissure to identify distal MCA branches on the surface of the insula. Next, overlying thick superficial Sylvian arachnoid membranes are disconnected.
Figure 7: The anterior Sylvian point, just inferior to the vertex of the pars triangularis is the most generous portion of the Sylvian cistern where the Sylvian split can begin. I use the distal deep opening within the fissure and identify the distal MCA branches as a landmark to further open the fissure from “inside to outside” or deep to superficial and from the posterior to anterior direction. I avoid the commonly used “outside to inside” technique, which is more difficult to perform because of the adherence of the frontotemporal opercula and lack of any landmarks to guide dissection of the interdigitating opercula. The superior Sylvian vein is preserved on the temporal side. Further details regarding Sylvian fissure dissection are discussed in the Techniques of Sylvian Fissure Split chapter.
After extension and completion of the Sylvian fissure dissection more medially along the distal M1 segment, the temporal and frontal opercula are mobilized away from the insula. The presence of the MCA branches between the temporal operculum and the insula facilitates early mobilization of the temporal operculum more than the frontal operculum. Occasional bridging Sylvian veins are coagulated and cut. At this point, proximal fissure dissection is extended to the level of the distal M1 segment and the LLAs to decrease the required amount of retraction on the opercula.
Distal fissure dissection is often limited because of adherence of the posterior opercula at this level; aggressive manipulation in this area will place the superior temporal gyrus at risk of injury. Gentle dynamic retraction of the frontotemporal opercula using the suction apparatus and bipolar forceps often provides good exposure of the insular cortex and the peri-insular sulci at each step of dissection. The tumor often violates the insular cortex and leads to adhesions between the opercula; dissection may lead to bleeding from the tumor surface and small short and superficial M2 perforators may be sacrificed early to allow for hemostasis and continuation of microsurgery. Aggressive indiscriminate coagulation in the face of bleeding must be avoided.
Sylvian fissure dissection alone provides a relatively narrow corridor for resection of most moderate to large size insular tumors. In addition, M3 branches often tether the frontotemporal opercula to the superior insula, further limiting elevation of the frontal lobe and undermining its opercula to remove tumor underneath the frontoparietal banks. These limitations are some of the most common causes of subtotal resection in surgery for insular tumors.
Since these tumors often own varying degrees of temporal or frontal extensions, additional cortical incisions or resections within the frontotemporal opercula are necessary to allow enough space to maximize tumor excision. Mapping of the patient’s face area (for nondominant tumors) or Broca’s and Wernicke’s areas (for dominant tumors) are necessary to further guide the location of the safe cortical incision(s) in the inferior frontal and superior temporal gyri. These maneuvers extend the working space and angles to facilitate adequate tumor exposure while minimizing the operative blind spots.
Before proceeding with tumor resection, the superior and inferior peri-insular sulci are generously uncovered.
Insular Tumor Resection
Figure 8: The M2 perforators on the insular cortex must be thoroughly coagulated and sharply cut to avoid injury to the parent vessel via their avulsion (left image). Avulsion injury of the parent vessel can result in its severe spasm and even occlusion, causing catastrophic ischemic injury to the distal MCA territories (right image). If minor injury to the MCA wall is detected, a small piece of thrombin soaked cotton may be placed over the defect and gentle tamponade by the patient operator will stop the bleeding. The small piece of cotton can then be removed. Papaverine-soaked gelfoam pledgets can relieve the spasm in the M2-M3 branches during their manipulation. Aggressive coagulation is not advised.
The lateral insular portion of the tumor should be removed before the patient is awakened. To accomplish this step, the short perforating M2 branches are sacrificed and the M2 branches mobilized to uncover the insular cortex infiltrated by tumor. Generous exposure of the insular cortex without aggressive retraction on the opercula is paramount. If the tumor involves the anterior part of the temporal lobe, a limited anterior temporal lobectomy can significantly expand the inferior-to-superior trajectory to allow tumor removal.
Figure 9: Once the perforating arteries have been coagulated and divided, several pial incisions can be made on the insular cortex between the M2 branches, thus creating several working windows/channels for subpial resection of the tumor. Inadequate mobilization of the M2 branches places these arteries at risk and compromises the extent of resection.
Figure 10: Intracapsular debulking of the mass is undertaken as the initial step in resection. The superior and inferior intracapsular margins of the tumor are estimated based on the location of the peri-insular sulci. The core of the tumor is removed using bipolar coagulation and suction. I complete a conservative intracapsular tumor removal and avoid handling the tumor margins until later in the operation when subcortical mapping and frequent neurologic exams are conducted under awake conditions.
I avoid ultrasonic aspirators in fear of MCA injury and use bipolar electrocautery and suction device to emulsify and remove the tumor, respectively. I always keep the MCA branches and lateral lenticulostriate arteries in sight to avoid their inadvertent injury by excessive retraction or manipulation.
The lateral lenticulostriate arteries are reliable anatomic landmarks to define the safe medial extent of the exposure and resection. They are usually displaced medially. Although the superior and inferior peri-insular sulci define the superior and inferior extent of the lateral operative corridor, these landmarks are often affected by tumor expansion and navigation is used to further orient the operator. The longer posteriorly and superiorly located M2-M3 perforators traveling toward the central lobule are strictly protected.
After the lateral portion of the tumor is removed, the patient’s sedation is allowed to wear off and subcortical mapping may be later used to guide the removal of the posterior and superior poles of the tumor.
Figure 11: A view of the resection cavity depicted through the trans-Sylvian approach for tumors mainly confined to the insula. The anterior and medial borders of the cavity are defined by the lateral lenticulostriate arteries (LLAs). The LLAs and the striatum (note its characteristic nutmeg appearance) are the two anatomic landmarks for guiding the medial margin of the resection. The corona radiata and the posterior limb of the internal capsule are located in the posterosuperior margins of the cavity. Further resection in this direction requires subcortical mapping and direct patient feedback (intraoperative exams) to avoid injury to these white matter tracts.
Figure 12: A right temporal lobectomy was initially completed for this insular tumor (inset image) and the MCA branches (*) have been mobilized. I carefully look for the LLAs (blue arrow), as any minor bipolar coagulation in their area will lead to their irreversible compromise. The characteristic nutmeg appearance of the striatum is evident in the background of these perforators. Following cortical mapping of the patient’s face area (marked with yellow arrow), the anterior inferior frontal gyrus was removed to expose the frontal extension of the tumor (see below).
Tumor removal continues with the aid of subcortical mapping. The region of the LLAs should be protected. The suction apparatus can easily injure these perforators. Any bleeding in this medial region should be controlled with gentle pressure of a piece of gelfoam soaked in thrombin solution.
Identifying the appropriate depth of resection can be very challenging, and the distal LLAs provide a reasonable landmark. Navigation can provide additional information at this juncture as the medial structures are least affected by brain shift. A highly magnified and well-illuminated surgical field through the use of the operating microscope and surgical experience can alert the operator to changes in consistency, color, and texture, which leads to clues about reaching the margins of the tumor in relation to the normal white matter.
Removing the posterior extension of the tumor into the posterior limb of internal capsule is one of the most challenging parts of the operation and should be performed patiently using subcortical mapping during the last operative stages. The resection should also be halted upon development of any minor reversible deficits.
Essentially, in addition to anatomical landmarks discussed above and the change in the texture and consistency of the tumor, resection is guided based on the following parameters:
- navigation “estimates” that the surgeon has reached the “normal” brain. The brain shift should be accounted for and can mislead the operator (often the plane of dissection is more lateral than indicated by navigation,)
- cortical or subcortical stimulation reveals functional cortices or fibers, or
- the patient experiences a minor neurologic deficit on intraoperative testing.
Operative efficiency is pertinent because most patients remain cooperative for approximately 3-4 hours under awake conditions. I do not use intraoperative MRI as it leads to a long operative session for the awake patient.
Additional Considerations
Larger tumors require expansion of the transsylvian corridor.
Figure 13: After initial removal of this left insular tumor (inset) through the trans-Sylvian route, the motor speech area was mapped (Le) on the inferior frontal gyrus. Additional corticotomy in the negatively mapped frontal opercula allowed further uncovering and exposure of the frontal and superior poles of the tumor.
Large Dominant Insular Anaplastic Astrocytoma
Figure 14: Resection of a diffuse dominant insular tumor through a combined trans-Sylvian and transcortical approach is demonstrated. The left and right upper images demonstrate the pre- and postoperative images and corresponding extent of resection. The lower photo illustrates the functional map in preparation for creating corticotomies within the inferior frontal and superior temporal gyri to reach the extensions of the tumor underneath the opercula.
Non-Dominant Insular Low-Grade Glioma: Principles of Resection
Closure
After hemostasis is achieved, the dura is closed while the patient is sedated. Watertight dural closure is not necessary. Tack-up sutures at the edge of the craniotomy and in the center of the bone flap should be used because of the large size of the craniotomy in order to avoid postoperative epidural fluid collections.
Postoperative Considerations
Seizure prophylaxis is important during and after insular glioma surgery; slightly supratherapeutic levels are often desirable because a number of functional cortices are manipulated during surgery and are therefore excitable. Drug levels should be measured before surgery and a strict dose schedule should be followed, including during surgery. A postoperative MRI is obtained and if significant residual tumor is detected (most often underneath the frontotemporal opercula or near the internal capsule,) a repeat operation is considered. Any areas of ischemia will prognosticate recovery in the postoperative period.
It is not uncommon that some patients develop deficits 24 to 48 hours after surgery, especially language deficits for dominant-side surgery. The etiology of such delayed but most often temporary deficits is not clearly understood, but postoperative edema and partial seizures most likely account for them. An EEG can be useful for detecting epileptiform discharges, and doses of anticonvulsant medications should be adjusted or new agents added accordingly.
The majority of patients who develop delayed deficits will recover within a period of 2-4 weeks. The family should be reassured. Steroids may be used during the immediate postoperative period, but should be tapered off quickly as the patient’s clinical status improves.
Pearls and Pitfalls
- The transsylvian approach requires comfortable familiarity with microsurgical techniques to generously dissect the fissure. Insular tumors often extend into the white matter and are covered by the frontotemporal opercula. An initial trans-Sylvian resection and subsequent extension of the transopercular operative corridor allows reasonable access to large insular tumors.
- Ischemic injury as a result of damage to the lenticulostriate arteries or middle cerebral artery branches is one of the main causes of surgical morbidity. To avoid these complications, the surgeon must have a thorough understanding of the regional anatomy and execute meticulous microsurgical techniques. Hemostasis and gentle handling of the vasculature are crucial. The long M2-perforating arteries leading to the corona radiata and central lobule should also be preserved and not coagulated inadvertently in case of excessive bleeding from the tumor.
- Injury to the descending motor fibers can be another source of morbidity and should be avoided via careful identification of distal LLAs more anteriorly and subcortical mapping at the level of the internal capsule more posteriorly. Aggressive resection can lead to significant morbidity.
- Another common cause of morbidity after the trans-Sylvian approach is excessive retraction on the opercula, which can injure the Broca’s area, the horizontal fibers of the arcuate fasciculus near the superior periinsular sulcus, or the fibers of the uncinate fasciculus near the inferior periinsular sulcus. To prevent this type of injury, I discourage the use of fixed retractors and encourage the use of the dynamic retraction technique. I use the suction apparatus and bipolar forceps to mobilize the opercula while constantly shifting the pressure points.
- The surgeon may have to rely on “negative stimulation mapping” without identification of unexposed or unmappable “positive” eloquent areas.
- Some insular tumors expand the insula and may protrude through the Sylvian fissure. This finding means significant adherence of the frontotemporal opercula to the insular cortex, making dissection difficult and placing the vasculature and functional cortices at risk.
Contributor: Roberto Rey-Dios, MD
References
Duffau H, Capelle L. Preferential brain locations of low-grade gliomas. Cancer. 2004; 100:2622–2626.
Duffau H, Taillandier L, Gatignol P, Capelle L. The insular lobe and brain plasticity: Lessons from tumor surgery. Clin Neurol Neurosurg. 2006; 108: 543–548.
Hentschel SJ, Lang FF. Surgical resection of intrinsic insular tumors. Neurosurgery. 2005; 57:176–183, discussion 176–183.
Lang FF, Olansen NE, DeMonte F, Gokaslan ZL, Holland EC, Kalhorn C, et al. Surgical resection of intrinsic insular tumors: complication avoidance. J Neurosurg. 2001; 95: 638–650.
Ribas GC, Ribas EC, Rodrigues CJ. The anterior Sylvian point and the suprasylvian operculum. Neurosurg Focus. 2005; 18: E2.
Ryvlin P, Minotti L, Demarquay G, Hirsch E, Arzimanoglou A, Hoffman D, et al. Nocturnal hypermotor seizures, suggesting frontal lobe epilepsy, can originate in the insula. Epilepsia. 2006; 47: 755–765.
Shelley BP, Trimble MR. The insular lobe of Reil—its anatamico-functional, behavioural and neuropsychiatric attributes in humans—a review. World J Biol Psychiatry. 2004; 5: 176–200.
Tanriover N, Rhoton AL, Kawashima M, Ulm AJ, Yasuda A. Microsurgical anatomy of the insula and the Sylvian fissure. J Neurosurg. 2004; 100: 891–922.
Türe U, Yaşargil DC, Al-Mefty O, Yaşargil MG. Topographic anatomy of the insular region. J Neurosurg. 1999; 90: 720–733.
Türe U, Yaşargil MG, Al-Mefty O, Yaşargil DC. Arteries of the insula. J Neurosurg. 2000; 92:676–687.
Varnavas GG, Grand W. The insular cortex: morphological and vascular anatomic characteristics. Neurosurgery. 1999; 44:127–136. Discussion 136–138.
Yasargil GM, Krisht AF, Türe U, Al-Mefty O, Yasargil DCH. Microsurgery of insular gliomas: Part III: pathophysiology and clinical presentation. Contemp Neurosurg. 2002; 24:1–5.
Yasargil MG. Microneurosurgery. Yasargil MG (ed), George Thieme Verlag: 1984
Yaşargil MG, Ammon von K, Cavazos E, Doczi T, Reeves JD, Roth P. Tumours of the limbic and paralimbic systems. Acta Neurochir. 1992; 118: 40–52.
Zentner J, Meyer B, Stangl A, Schramm J. Intrinsic tumors of the insula: a prospective surgical study of 30 patients. J Neurosurg. 1996; 85:263–271.
Please login to post a comment.